
How to tackle manufacturing planning for cell and gene therapy?
Cell and gene therapies are here, and they’re here to stay. In the last couple of years, very promising results have been achieved. Examples include treatments for certain blood cancers that helped patients for which all other available therapies had failed. Another example is a treatment for a genetic mutation that causes blindness. However, the process for manufacturing these therapies is complex and unique. Our VP of Product, Joachim Lasoen, explores this and outlines why it’s important to implement a dedicated cell and gene therapy software product that has been designed to address those challenges.
How does CGT work?
An example is CAR-T therapy which is in essence (without trying to be complete or scientific) something like this:
- Cells are harvested from the treated patient
- In the lab, cells are genetically modified and cultivated
- The manipulated cells are re-administered to the patient
- The manipulated cells can then recognize and destroy tumor cells in the patient and initiate a broader immune response.
Manufacturing planning – new challenges ahead
The novelty of highly efficacious but highly complex and expensive cell and gene products is disrupting every element of the route to commercial success including supply chain needs, reimbursement models, and more.
Manufacturing processes are completely different from what the life sciences industry is currently used to. Whereas in standard manufacturing big batches are produced to stock, cell and gene therapies manufacture batches of 1 (per patient).
These batches need to be produced in a very limited timeframe. When cells are collected from the patients, it is critical that the cells have somewhere to go, that there’s manufacturing capacity available.
It’s also about scheduling and balancing cell collection with manufacturing and infusion dates to have a more efficient supply chain, which should impact favorably on the cost of those therapies.
One could question why the conventional leading solutions in supply chain and production planning and scheduling (APS software solutions such as OMP, Kinaxis, SAP APO and others) cannot be used. At Bluecrux we implement such solutions in commercial supply chains. Such solutions thrive on footprint and robust processes. That is not what CGT is. We confirm that there is not a single conventional supply chain concept that cuts it for CGT plants. To meet planning and scheduling requirements, we are talking about a radical conceptual change.
Did you know that Bluecrux has published an eBook about the CGT industry?
Introducing
Voices from the Advanced Therapies Space: collected discussions and advice from experts in the CGT industry
A unique deep dive into the challenges of the cell and gene therapy sector, from the perspective of established experts in the field, in their own words
Download your FREE copy today!How to tackle planning and scheduling?
Through three implementations that started in 2018 in the domain of lab scale production, we discovered that Binocs covers quite some of the planning and scheduling requirements. Extending Binocs to CGT is a linear step:
- Binocs resource planning and scheduling run in different research-scale cell culture environments (Merck, GSK, UCB, …)
- Many of the cell and gene therapy manufacturing processes run currently as scaled-up versions of the cell culture processes. The steps described below are based on our experience in these environments.
- However, the CGT manufacturing processes are changing rapidly. We’re rooted in co-creation and are starting projects with clients to enhance Binocs to meet the unique needs of CGT environments even better.
1. Plan for growth
As demand for patient treatments is increasing, so will the need for a robust and industrialized process. Asset utilization of clean rooms and bioreactors will become a challenge as it requires a delicate interaction between processes and qualified technicians. Therefore getting control through a tight planning process is required:
- Using the Binocs equipment planner different batches are planned and equipment reserved.
- Planners can run what-if scenarios and test different plans
- Planners stay in control and update the planning, but get immediate feedback of overbookings or periods of over/under capacity.

Binocs covers end-to-end activities. This way planners can promise manufacturing slots, which in turn triggers the upstream processes (doctor, patient, logistics) to plan the cell harvesting accordingly.
2. Scheduling technicians
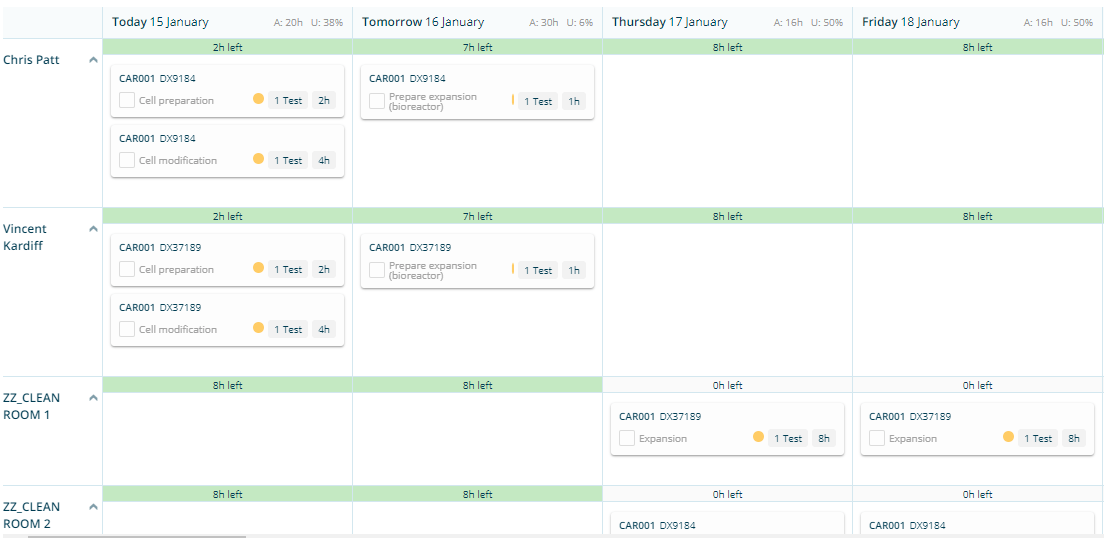
The different manufacturing steps are configured in Binocs with the correct timings and lead times. Based on this configuration the Binocs algorithm will schedule technicians and equipment taking into account qualifications, availability and other constraints.
This will give the technicians a one-stop overview of their planning on the Binocs planboard.
3. Plan the Quality control in one system
Binocs is the leading in the domain of QC resource planning and scheduling. So not only the manufacturing department, but also the QC analysts can be planned using Binocs. The different QC tests (e.g. PCR sterility test, mycoplasma) can be configured similarly in Binocs and scheduled on the plan board. As also QA release activities, reagent and media preparation, validation activities, etc can be covered, Binocs can be considered as the digital twin of the CGT plant. All the activities in and around the CGT plant are visible in one system: the past (actuals), the present (progress status) and the future (planning).
4. Interfacing with other systems
The visibility of the entire cell therapy process — from initial material collection to final treatment, is the new challenge. We need to avoid the time, cost, and risks associated with outdated paper-based systems. Therefore manufactoring Execution Systems (MES), Logistics Systems as well as lab systems (LIMS/ELN) are needed by which program-critical information can be collated, tracked, and documented.
System such as Vineti or TrakCel offer platforms to do this. CGT manufacturing planning software such as Binocs is specifically designed to send and receive data to these platforms to give real-time visibility on manufacturing planning.
Based on our experience at clients in Cell culture, Clinical trial and QC departments we’re expanding (in co-creation with leading pharmaceutical companies) the resource planning and scheduling software Binocs to fully support Cell and Gene therapy manufacturing planning.
Joachim Lasoen
Joachim is Head of Solution for Binocs.



