
The future of CGT: the challenges of scaling operations
In this article, cell and gene therapy (CGT) specialist, Akshay Peer, outlines the current challenges facing advanced therapy developers looking to scale up their interconnected supply chain and manufacturing operations. He explains why the ability to link demand planning, scheduling, and manufacturing execution is not merely a value enhancer, but is a core pre-requisite for the future of CGT products, especially autologous therapies.
The current state of the CGT industry
Next-generation therapeutic products, including CAR-T and AAV-based, are already on the market and being used to treat previously untreatable conditions. These products are the future of healthcare, with more than 1,300 trials currently underway with more than 90,000 patients treated in these trials (ARM report 2020). EY predicts the global CGT market will experience a meteoric rise, from an estimated US$3bn annually in 2021 to an estimated US$50bn annually in 2027.
As more CGT therapies scale-up, execution and planning of pre/post treatment activities, logistics and manufacturing operations will require full visibility of end-to-end available capacity. Demand and supply management will be more similar to airline flight operations rather than a push type of manufacturing system
Vision and aim for the CGT industry
Despite the exceptional therapeutic and potentially curative nature of cell and gene therapies, these are prescribed at a very fragile point in a patient’s treatment. Ensuring each patient receives their drug product in a timely fashion is critical for patients and their families. Exceeding patient and caregiver expectations should be the baseline of operational setup adopted by companies/manufacturer delivering these therapies
- Collection of the starting material (apheresis, bone marrow or tumor biopsy) should be convenient and performed as scheduled
- No patient should need to return for another collection (unless necessary) due to inadequate manufacturing capacity or logistics mistakes
- Health Care Providers (HCPs) are busy, especially in the recent turbulent times, minimizing disruptions to their schedules is essential. Providing visibility of most feasible starting material collection (in line with manufacturing capacity) is key to providing high customer satisfaction and service levels.
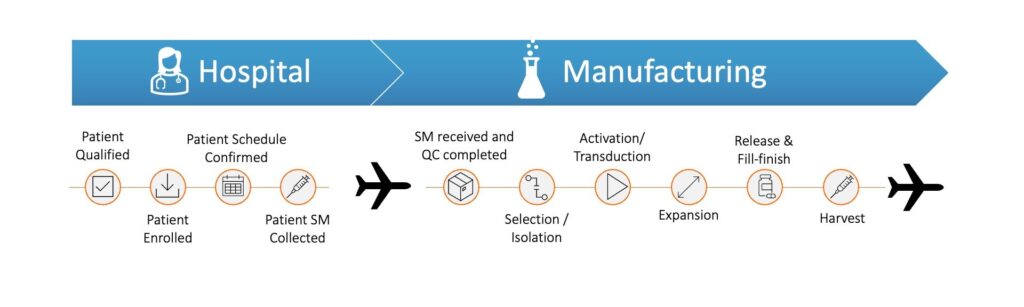
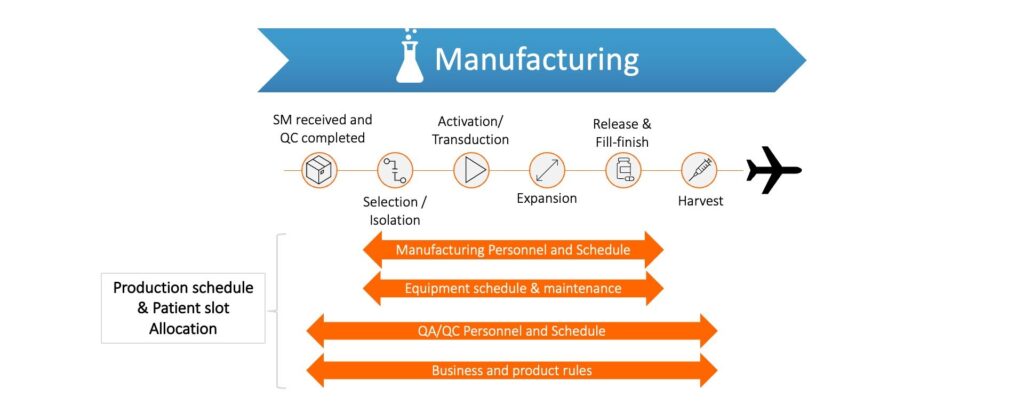
For autologous therapies, cancer vaccines and matched allogeneic therapies coordination across multiple parties and an integrated eco-system is critical to delivering these life-changing therapies. All therapy developers need to aim at a high level of service to their patients and make decisions on investing in an appropriate operational footprint. The desired level of service will define the strategy for scaling up – centralized, de-centralized, fresh or frozen product, etc.
Achieving all of the objectives requires manufacturing and supply chain to work in harmony as decisions are taken throughout the therapy development process; after all, therapeutic development is a game of resource/asset allocation and capacity management.
Did you know that Bluecrux has published an eBook about the CGT industry?
Introducing
Voices from the Advanced Therapies Space: collected discussions and advice from experts in the CGT industry
A unique deep dive into the challenges of the cell and gene therapy sector, from the perspective of established experts in the field, in their own words
Download your FREE copy today!Centralized manufacturing for CGT products
Biopharma and pharmaceutical companies have invested heavily in building or acquiring manufacturing capabilities with the likes of Novartis, Gilead, Bristol Myers Squibb (BMS), Iovance, Adaptimmune, CRISPR Therapeutics, and many others building their own manufacturing capabilities to service their clinical and commercial products.
Contract Development & Manufacturing Organizations (CDMOs) are also investing heavily in creating state-of-the-art facilities to deliver therapies at scale. Major market players like Lonza, Catalent, Thermofisher, Charles River have acquired and/or built out manufacturing operations across multiple regions. Since 2016, there have been about 20 M&A transactions worth more than $30 billion in the CDMO community alone.
Even with increased investment and more manufacturing facilities available than ever before, early CGT therapy developers are on waitlists to attain manufacturing slots and opt to procure services from multiple CDMOs in an attempt to expand their possibilities for treating patients as and when required. Due to this volatile and high-demand scenario, companies are raising further funds to invest in their own manufacturing facilities for both vector and batch manufacturing. This approach is great to take control of the supply chain and planning but comes with its own challenges
1. Production and QC Scheduling
Production and QC go hand-in-hand in developing a drug product. Manufacturing managers and production planning leads for a therapy developer must optimize the utilization of manufacturing capacity and resources for patient treatments. It is imperative to manufacture as many therapies as possible to give more patients access to potentially life-saving treatments. Ensuring the people, equipment, materials, and QC teams are available for batch manufacturing operation is essential before scheduling the collection of starting material.
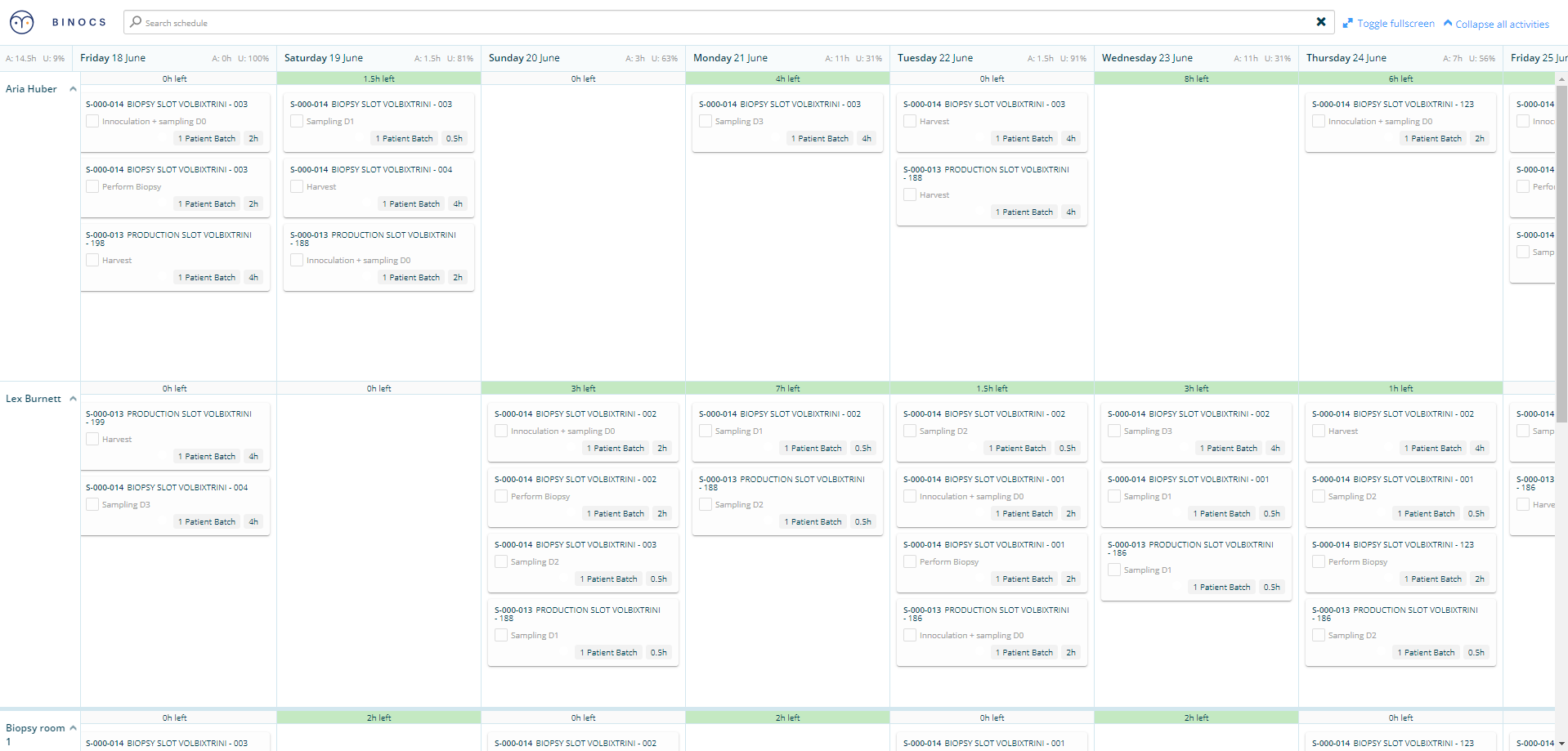
Most companies use ‘white boards’ or manually updated excel sheets in managing production schedules. Quality and other teams have their own Excel sheets that need to be manually updated each time anything changes across their teams. This includes changes in testing schedules, availability of key personnel, equipment downtime or maintenance, etc. Harmonizing the schedules across the disparate teams is challenging and time-consuming
2. Resource planning and allocation
CGT industry has a shortage of specialized workforce that can be quickly recruited and utilized for manufacturing. Most companies are hiring people with biologics or similar backgrounds and training them further to support their operations. However, due to the high employment costs and lack of a trusted pool of personnel that is readily available, scheduling manufacturing runs can be a challenging task. As more personnel are added, different resources might have varying levels of competence and training. Scheduling competent and trained resources is an integral part of CGT manufacturing planning.
Training of the personnel records is maintained in Quality systems (paper-based or electronic). These records require continuous updating, and personnel training levels have to be communicated to the production planners to schedule accurately. Manually cross-checking competencies of critical resources is time-consuming and tedious.
3. Equipment allocation and maintenance
Similar to resource allocation, equipment or cleanrooms are required for producing a single batch of the product. In most manufacturing runs, equipment like bioreactors can be utilized for a few days and can be a bottleneck for scheduling new manufacturing runs. Different equipment used during each run may also require maintenance, sterilization time, calibration, etc., at varying schedules. The manufacturing planner or manager needs to know which equipment is utilized or ‘out of service’ to plan and schedule manufacturing runs accurately.
4. Patient slot allocation
Based on all the above-mentioned factors (internal capacity and schedules), supply chain teams need to calculate and know when a patient’s material can be collected and shipped for manufacturing. If the incoming starting material is a fresh product (24-48 hour shelf life), then ensuring manufacturing capacity availability is critical for patient treatment.
Therapy developers utilizing frozen incoming material (cryopreserved) have more flexibility in starting manufacturing but it still requires them to move fast and create the drug product in a timely fashion as most patients are gravely ill. In some cases, further delays in receiving their drug product could prove to be fatal. Therefore, calculating an optimal slot for manufacturing and communicating this to HCPs is essential to create a harmonized supply chain for CGT products.
5. Re-scheduling happens more often you think
Confirmed schedules can change due to several reasons, including patient’s health and availability for collection, manufacturing or logistics issues or QC unavailability, etc. We have seen nearly 30%-40% of confirmed schedules change and require re-scheduling.
As a supply chain planner, it is essential to know and communicate to HCPs when the next slot for collection is possible. Calculating the next optimal slot might be simple in a conventional single-product manufacturing line, but not in CGT. The inherent variability in the patient’s starting material impacts the manufacturing cycle times leading to significant variances between batches. Consequently, calculating the next available manufacturing slot will require a manual check across multiple teams and assets.
Manufacturing and supply chain teams need visibility into the correct and timely information to continually monitor the status of scheduled manufacturing, including analyzing inbound orders and tracking new ones. At the same time, optimizing availability and managing the overall schedule on the manufacturing floor.
Future of CGT manufacturing
In the long term, automation of the manufacturing process by companies like Cellares, Octane (Lonza) and Ori-Biotech are in the works. However, no company has produced ‘multiple’ batches for even clinical studies, let alone commercial products. Some companies are also keen to develop de-centralized manufacturing scenarios where the drug product is created near the patient. Still, these will require more time, training and innovation before becoming a commercial reality.
In the short to medium term, biopharma companies and CDMOs will continue to expand their manufacturing footprint across multiple geographical territories. Most biopharma companies especially want to be self-reliant and control their processes before commercializing the lead candidates. Once a product is commercial, there will be additional pressure to balance between multiple clinical programs and commercial production. Managing this complexity and offering a high level of service to biopharma clients (hospitals, nurses, doctors) is critical for the success of their products and patient enrolment in clinical studies.
Scheduling at the heart of CGT operations
To improve biopharma companies’ responsiveness, eliminating expensive waste caused by disruptions in patient schedules or other supply chain and manufacturing exceptions, companies will need to invest in appropriate and state-of-the-art art systems or tools. Managing internal manufacturing schedules across multiple locations, teams and competencies will be paramount to deliver a high level of service to its clients. Companies with manufacturing facilities will need to invest in talent who can manually manage the complex and ever-changing schedules—or adopt a CGT-specific scheduling tool.
This tool will help the manufacturers manage and optimize internal capacity across different teams, protocols/products and offer Just-in-Time (JIT) scheduling of patient’s starting material collection.
Looking for a CGT-specific scheduling tool? Click here to have a chat and see what Binocs can do for you!
Akshay joined Bluecrux as the CGT lead in 2021 after a distinguished career as the co-founder of TrakCel (now the market leader in providing CGT orchestration solutions). With more than a decade’s experience in supporting advanced therapy manufacturers involved in CAR-T, TILs, Tregs, CRISPR Cas 9, and other similar ‘made-to-order’ therapies, Akshay has worked closely with the Binocs Product and Solution teams at Bluecrux to engineer a planning solution that is tailored to the specific needs of this challenging industry. Akshay also leads the IWG for Binocs and actively participates in industry events and thought leadership discussions.



