Planning your QC lab with APS software: is it a good idea?
Advanced planning and scheduling (APS) tools such as OMP, Quintiq, Ortems, Preactor, bioG, etc. are designed to help manufacturers plan their Production processes. As such, APS software focuses on optimizing production fulfillment, safeguarding service levels, and managing inventory in the network. At Binocs, clients in quality control often ask us: “if we already use APS software to plan and schedule our production processes, it must make sense to use the same software to plan and schedule our quality control labs, right?”. In this article, lab software specialist, Frederik Jaenen, outlines why this might not be the best idea.
APS to plan your QC lab: why not?
The assumption is that having everything in one system enables you to make more informed decisions and have immediate feedback on specific planning scenarios. For example, a potential stockout is imminent, could we expedite the production schedule as well as the release in the quality environment to still serve the market in time?
However, it’s a trade-off: there’s typically a significant configuration effort needed to make the advanced planning and scheduling tools fit-for-purpose and perform as they should.
“The more immediate gains are in streamlining the alignment and decision processes between the players and significantly uplifting the capabilities in the quality teams to provide feedback on requests with more accuracy through best in breed solutions—specifically designed to provide those answers.” Explains Cedric Van Helleputte, Head of Planning for Life Science at Bluecrux.
So, the short answer: you could use APS software to plan your QC lab, but is it really what you want?
Production Planning VS Quality Control in Laboratories
Planning production is very different from planning a quality control lab. Here are the three main differences:
1. The timeframe is different
Production environments tackle mid to long-term planning and move into the short-term horizon with a focus on making decisions such as ‘which batch should I produce on which bottleneck machine to fulfill my customer demand on time?’
However, APS software is not tailored to steer operations or prepare the shop floor for actual production. It works with frozen fences and, for example, handover the detailed schedule a week upfront to production.
“In QC Labs, the horizon of decision making is different, and we want to make decisions that closely intertwine planning and execution,” insists Cédric. “It’s a very different puzzle, which requires a planning process that is flexible enough to quickly adapt, re-prioritize, re-shuffle… on the very short-term.”
2. Capacity planning has different constraints
In a production context, the status of materials basically defines capacity constraints. For instance, do you have sufficient materials to do what you need to do (raw materials, packaging, etc.)? Thus, production capacity management asks “how do we make the best use of the available capacity to meet customer demand?” and “how do we place smart inventory buffers to ensure customer coverage is guaranteed?”
The capacity of a QC lab, on the other hand, is not a function of materials and production capacity. It’s a matter of having the right equipment and the right analysts (with the right skill set), all available at the right time.
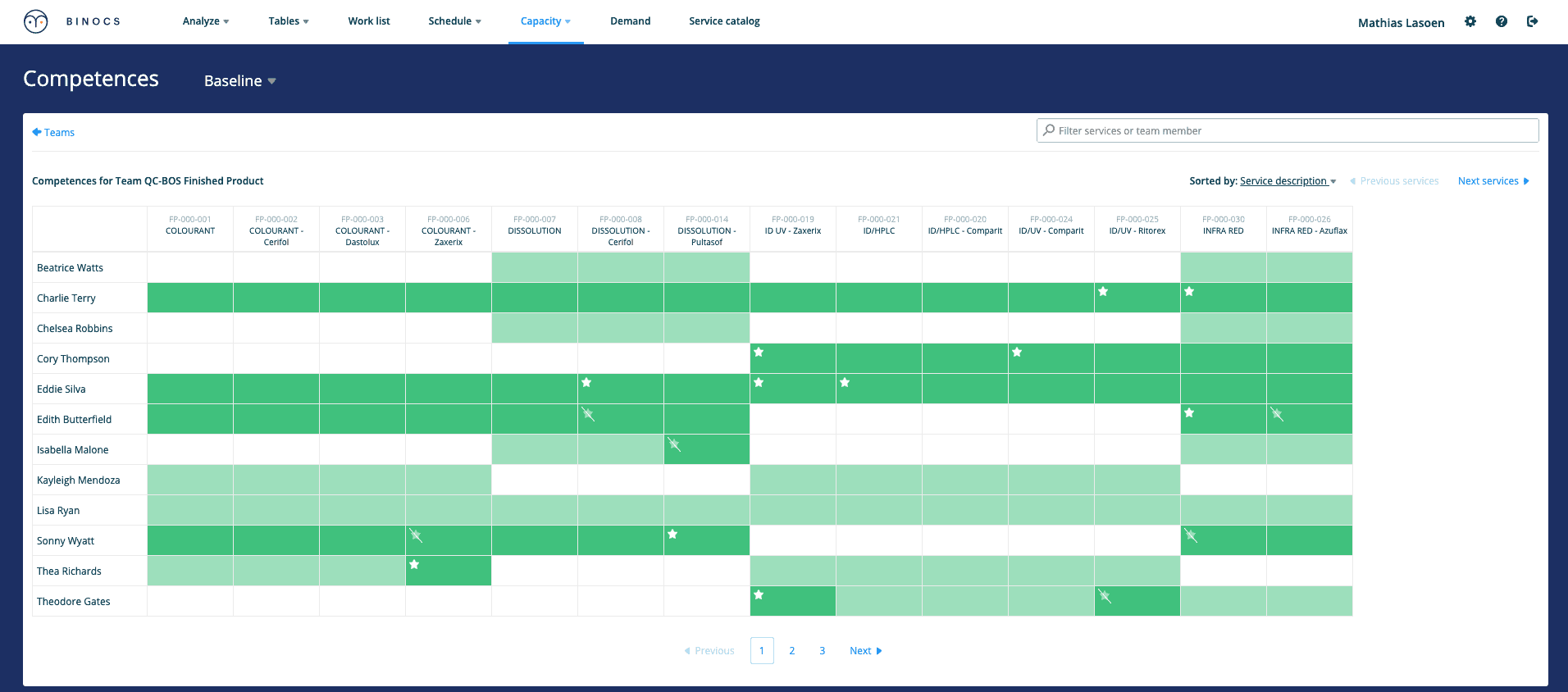
Skill and qualification management is crucial in a QC lab: having the right skills (i.e. competencies) available at the right time. Skills can vary in time and need to be configured in detail to optimize planning and scheduling fully.
Lab scheduling is also primarily about human beings. Lab managers and schedulers have to consider work variation and preferences to keep the staff sharp and motivated. APS software typically doesn’t cover this level of detail, simply because it doesn’t have to: the focus is just different.
3. Opportunities for optimization are different
In both production and quality control labs, one of the critical opportunities for optimization is campaigning.
However, whereas campaigning in production refers to minimizing set-up and cleaning times of your production lines (through smart planning), in quality control labs, it refers to grouping samples in ‘runs’ for simultaneous processing.
QC labs require sophisticated business rules (higher level of granularity) and very specific constraints, as well as take into account the laboratory’s realities such as the physical aspects, failure ratios, etc.
Challenges—feedback from the field
“When implementing APS, a lot of effort of the supply planning goes to modeling the right granularity to support planning decisions,” explains Cédric “like how to deploy available capacity, set inventory buffers to best meet patient demand.” This is deliberately a lower level of granularity than the shop floor reality to increase decision-making speed.
To steer planning in quality labs, you need a higher level of granularity.” And that’s because, compared to commercial production environments, execution and planning are highly intertwined. Therefore, APS software typically needs to close a number of gaps through programming or advanced configuration in order to match it to the needs of a QC lab. Four examples to support that point:
1. Need to configure competencies
As mentioned, in order to use advanced planning and scheduling software in a QC lab, you’ll need to configure the way you manage your competencies. Skills can vary in time and need to be configured in a lot of detail (not only on test method but also on activity level, for example, ‘test review’).
2. Need to configure campaigning
Many consultants consider automated campaigning as a key driver for optimizing utilization rates. But as lab methods change, new products come in,… it’s important that the business rules for campaigning are configurable by key-users. Otherwise, you’ll be back to campaigning manually quickly.
3. Need to accept sub-optimal user interface
A number of production challenges are not relevant or focused on QC lab constraints, resulting in non-optimal user interfaces that require heavy customization.
4. Near real-time scheduling
Every QC lab copes with process dynamics that require rescheduling and re-campaigning samples and tests based on production events and changing supply chain priorities.
The limits of advanced planning and scheduling software
Even after significant configuration, APS software is still APS software. Matching demand and capacity of a QC lab requires a certain level of granularity that commercial APS software does not offer.
The layer of configuration on top of the key building blocks is insufficient to cover the particular principles of efficiently planning a quality control lab.
Non-value-adding features
While in a production environment, it makes sense to model your plan on a Gantt chart based on key constraints (production line 1 will produce x quantity between 11 and 12). Typically, that’s not how you want to plan a QC lab, as there is a lot more variance in the execution.
Would it work? In theory, yes. Will it add value? Probably less than you’d hope. Furthermore, clients tell us that the stability of the lab’s schedule (during the day or shift) is key to safeguard lab analyst focus, efficiency & satisfaction.
Lacking features
You can add algorithms to campaign samples but adding conceptual aspects in a key-user friendly way is difficult.
Think of how you’ll manage detailed definitions of the lab’s lean way of working? Or the in-depth configuration of your analysts’ competencies? Or how you’ll handle the matrix way of working, typical for QC labs: multiple sources (product types, RM / IPT / release/stability, ad hoc samples…) to be assigned to various lab sections. Making all of these things work is not something to take lightly.
Scheduling the full picture is a challenge
In most QC labs, release testing, linked to production, is 50% or less of all the work a lab does. Other demand sources are stability testing, standards and reagents management, validation, qualification, R&D support, and the like. Each channel of demand has specific characteristics in terms of scheduling horizon, how the demand is expressed etc. APS systems are not designed to handle this.
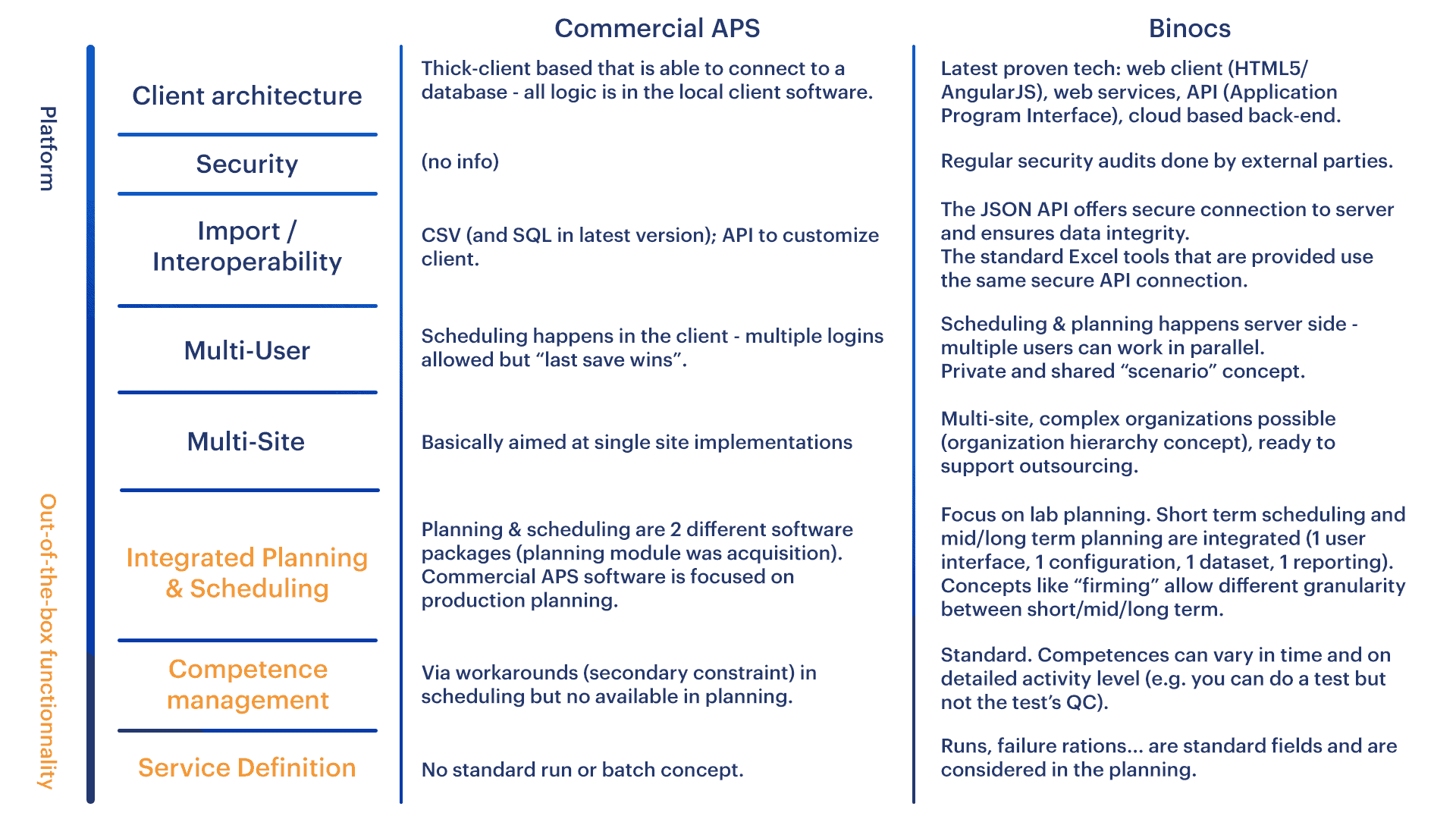
Commercial APS and QC lab-specific Scheduling Software Comparison
The summary table made by one of our customers makes some of the aspects we’ve explained more tangible.
Capacity planning and demand management software for QC Labs?
Discover Binocs today!
Implementing an advanced planning and scheduling system in a lab requires programming elements that are inherently foreign to make it work. Gartner and other industry leaders also warn from using ‘custom software’ because it is expensive to upgrade, even the smallest of future modifications require external expertise, and it’s not scalable.
Therefore, you probably want to look for a more out-of-the-box system, built specifically for QC labs, and preferably one that is enterprise-ready and in the cloud. Nothing forced, easy to deploy, and an open road for future updates & rollouts, like Binocs.
Frederik is VP of Sales at Binocs and has over 25 years of experience in laboratory systems, including as a Project Manager for major LIMS rollouts at various global enterprises. When he joined Bluecrux in 2017, his experience and business development skills significantly boosted the commercial success of Binocs.