
Analytical Method Validation: developing your mission control center with Binocs
In Part 1 of this series of articles on Analytical Method Validation, Adam Lester-George discussed the ways in which it shares key features with space missions. In Part 2 he describes how you can set up your lab’s very own “mission control center” using the Binocs resource management system as a form of analytical method validation software.
What is Analytical Method Validation?
QC laboratories do far more than perform release and stability testing. In fact, while such business-critical tasks are high-priority and must be delivered to strict schedules based on client requirements, they typically represent only about 60% of the work for which a lab is responsible. The remaining 40% is composed of secondary lab activities (often called indirect work) and includes critical work such as equipment calibrations, standards and reagent management, R&D support and analytical method validations.
In brief, Analytical Method Validation represents a set of clearly defined checks that are designed to ensure that the processes followed for each test method are fit-for-purpose, meet regulatory standards, and can be performed in a reliable and repeatable fashion (over time and between analysts). Ultimately, method validation is the quality assurance of the quality control processes themselves!
Challenges of planning Analytical Method Validation
Prioritization
Secondary lab activities must be planned around the value-add work of method testing but must often also be performed at regular intervals and within specific time windows of their own. Indeed, due to regulatory and GxP requirements, such tasks are critical to the continued ability of lab technicians to reliably conduct their primary testing responsibilities. Although such activities are secondary to the core processes, they nevertheless must be prioritized proportionately and so should not be left to ad hoc planning.
Whereas equipment calibration and environmental monitoring activities can generally be planned months in advance with a predetermined frequency, validation work is triggered in a variety of different ways. This can present significant challenges when attempting to reliably plan work around the lab’s primary functions.
Forecastability
Validation triggers range from periodic re-validation (which can be quite predictable and planned far ahead) to the introduction of new products – and consequently test methods – or changes and optimizations to existing test methods and equipment (which may have significantly shorter lead times or involve more intensive or disruptive work).
As a result, method validation rarely represents a flat workload that can be netted from the gross capacity; a “lumpy” demand profile, where there is a large variation in the quantity, frequency, and predictability of work, is very challenging to reliably forecast and plan without support from a resource management system.
Sequencing
Method validation typically follows a specific work pattern with contributions from different teams. For instance, the validation sequence will typically always begin with the development of a validation protocol, in which the Validation team defines exactly which validation activities must be performed in what order; the protocol will then be passed to the lab team, where technicians will need to plan first familiarization and then actual validation runs (including necessary adjustments and re-tests) in accordance with the documentation; after which, the final validation report can be prepared to outline the outcomes and officially record the completion of the specific testing.
While protocols can potentially be written some time in advance, the validation activities themselves will typically require specific and restrictive timelines. The complexity of following this process for every single test method means that most labs need to work on a Validation Master Plan (VMPs) that has been compiled by their QA department. A VMP defines not only the order of tasks but all of the potential variables (staff members, training, locations, processes, products, documents, etc.) that are required for delivery, as well as how the requirements for different method validation processes might interact or conflict with one another. In addition to writing and maintaining the VMPs, simply following up on each separate team/lab’s execution rapidly becomes is a highly intricate and time-consuming activity for the QA team.
Resource allocation
As briefly mentioned in Part 1, there are standard validation characteristics (such as accuracy, precision, linearity, detection/quantification limits, etc.) that should be tested as part of any method validation. Such testing requires a range of equipment and materials and a variety of qualifications/skills. This generally means that not all of the relevant tasks can be assigned to a single individual or team of analysts but must instead be distributed efficiently across the individuals with the appropriate expertise. Likewise, it requires that specific items of equipment be reserved for critical tasks when they might otherwise be needed for method testing, further contributing to the complexity of planning and scheduling.
Dynamic scheduling
One of the biggest challenges faced by any Planner when organizing lab activities is the management of last-minute changes, exceptions, and derivations in a dynamic and responsive fashion. For validation activities specifically, complex planning that has been prepared in advance to fit around primary lab activities and resource availability can be thrown into complete disarray by unexpected complications, such as unforeseen staff absences, urgent re-test requirements, or equipment failures.
Often, a bottleneck in an upstream process or even in an entirely unrelated set of activities elsewhere in the lab can cause a chain reaction that results in the right person with the appropriate training no longer being available to deliver a critical step in the validation sequence. Adjusting the short-term planning schedule to accommodate these exceptions can often lead to hours of frustration and lost productivity by the Planner as they struggle to optimally rebalance the workplan across myriad, highly complex, and interacting requirements.
Co-bot resource management with Binocs
The Binocs resource management system acts as a “co-bot.” This means that, based on the master data that your team carefully defines, the system can readily automate the processes that are simply too complex for a human brain to handle effectively while also allowing your human planners to make manual changes as required. In this way, the labor-intensive processing is “outsourced” to the algorithms in a collaborative fashion that gives human ingenuity and dynamism the final say on planning and scheduling decisions.
What follows is a very brief overview of how this co-bot approach can be implemented to effectively plan Analytical Method Validation activities.
Standard validation processes with Binocs
In Binocs, all test methods can be defined according to a Standard Work approach, with activities organized in a specific sequence and with predetermined interdependencies and resource requirements. Setting up Standard Work is easy and flexible and allows for the steps involved in a given method to be clearly defined, thus improving the method repeatability over time but also readily identifying the individual steps that will later be required for validation.
Indeed, not only can the test methods be defined as Standard Work services, but so, too, can the analytical method validation procedures themselves. Because a Binocs Standard Work can be composed of an unlimited number of independent and interdependent activities, it represents an ideal building block in dynamically planning validation work.
For instance, the Standard Work for a generic analytical method validation service (protocol writing) might be composed of the following activities:
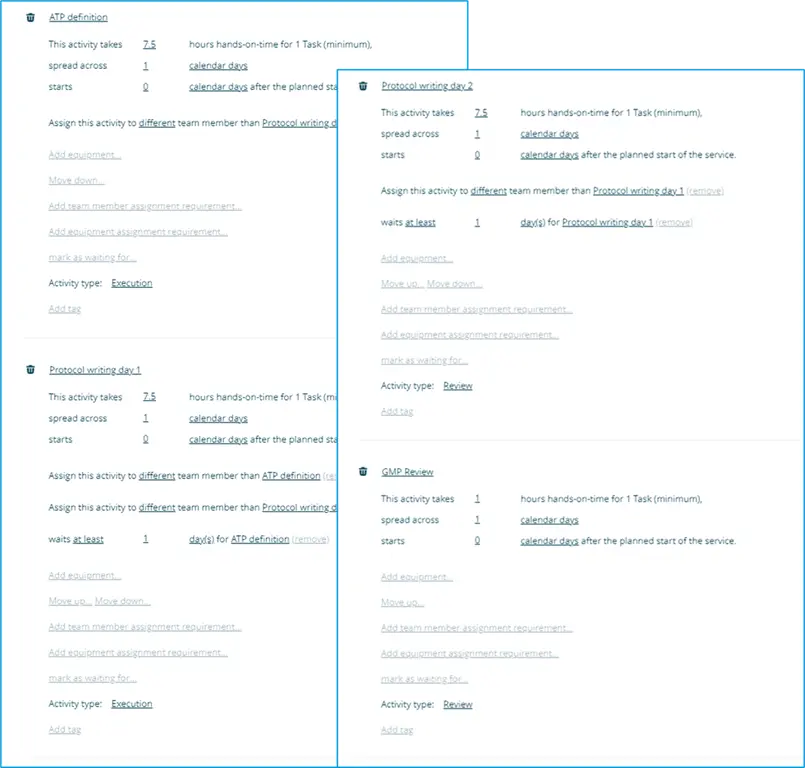
Because each activity is clearly defined, the Binocs system can then forecast the validation work in advance, planning for the time required and also allowing each constituent task to be allocated to an appropriately trained individual, specific items of equipment to be reserved ahead of time and for everything to be scheduled accordingly, down to the activity level.
Defining validation expertise with Binocs
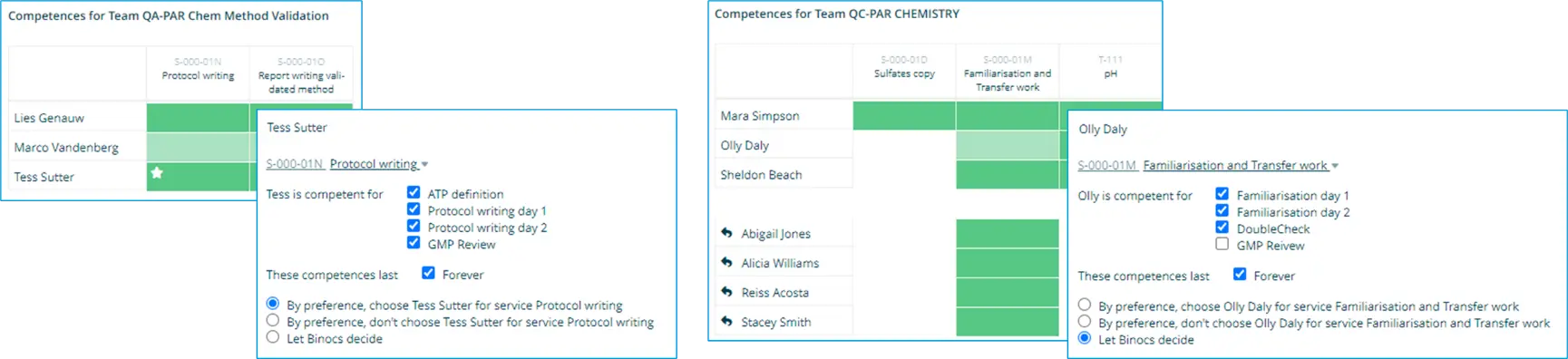
Binocs allows you to define and maintain a detailed matrix of team member competencies so that the training and expertise of your technicians can be easily tracked. Because competencies are defined at the level of the individual activity, team member allocation for validation tasks can be both highly specific (with the system selecting the best person to perform a given activity) and highly flexible (allowing last-minute substitutions to be identified if the first choice is unavailable).
This also means that the resource allocation for your primary lab activities can be planned efficiently, thus reducing the impact of exceptions and decreasing the frequency of bottlenecks in other parts of the lab that might have a knock-on effect on validation processes (and vice versa).
For instance, the below demonstrates detailed competencies for tasks performed by both a QA Validation team (Protocol writing) and a QC Chemistry team (Familiarization runs):
Planning validation procedures with Binocs
In Binocs, all work is defined according to which services (and therefore which sets of activities) are required to meet a specified demand. Such work is organized in a Demand Channel, where blocks of work can be organized according to the required time window (requested start à the requested end date).
Demand can be added manually on an ad hoc basis or, through Binocs zero-code interfacing, via external sources (such as LIMS or another sample management system). Demand can also be created according to a predefined operating model, with complex interdependencies between services and sets of tasks set up in advance and triggered by an external event – for instance, a new product introduction. By combining the operating model (OMM) with specific business rules to prioritize validation tasks, Binocs constantly updates validation workflows based on the VMPs.
Scheduling validation procedures with Binocs
One of the most significant benefits of the Binocs co-bot approach is in reducing the cognitive load on planners when it comes to the dynamic scheduling and re-scheduling of lab work. Because primary and secondary lab activities rely on a shared workforce of skilled individuals – and because Binocs knows who has which skills and availability on any given day – validation tasks can be readily scheduled and leveled together with all the other lab work.
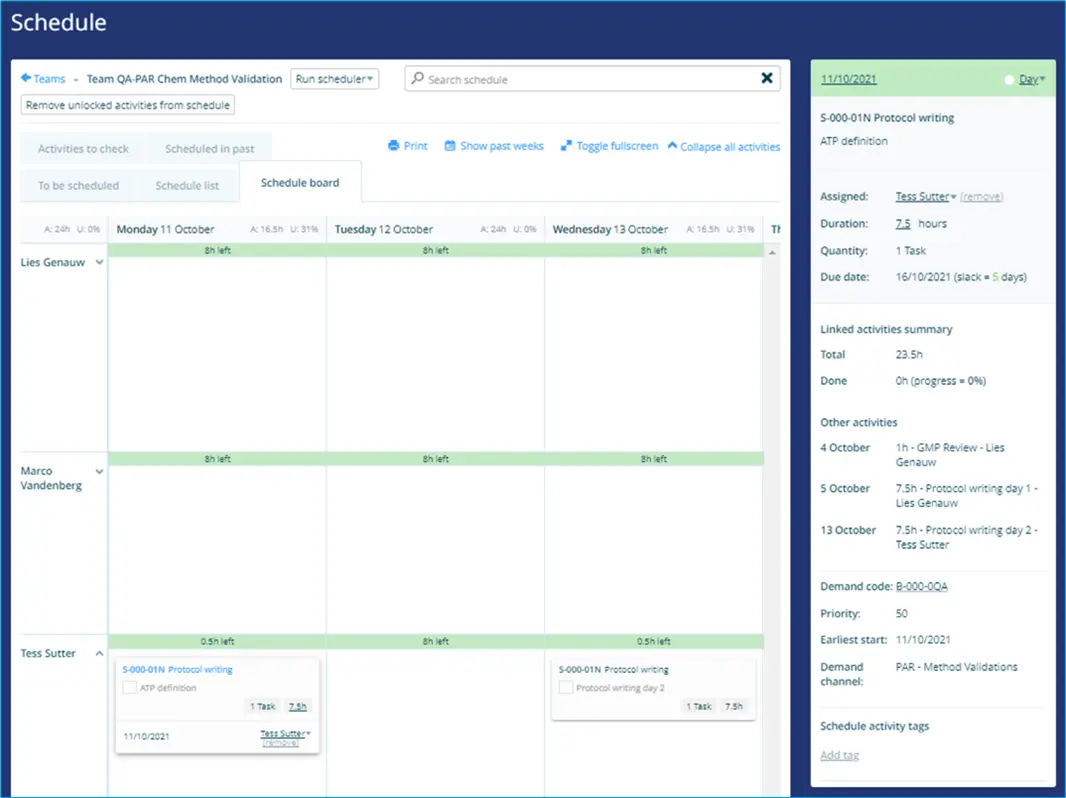
Below demonstrates the scheduled Protocol writing (design) task of a QA Validation team:
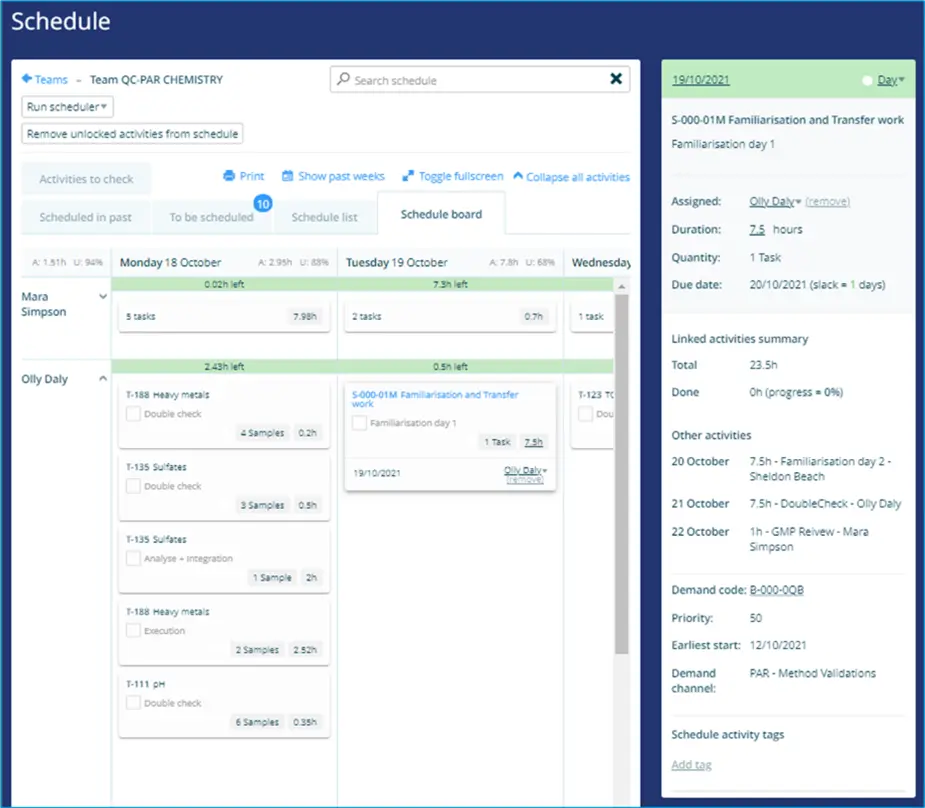
Below is an example of some testing work for a new method validation, scheduled in an optimal way with the release and stability work for a QC Chemistry team:
This allows for excellent and responsive management of both the highly lumpy demand profile and ad hoc exceptions and absences. Finding the optimum schedule when a validation test needs to be repeated or in the face of an unplanned resource shortage no longer requires hours of manual re-leveling, trial & error, and best-guess allocations – with Binocs, all it takes is a few clicks to reorganize the workplan automatically, while still allowing for manual refinements and sign-off by the Planner themselves.
Analytical validation method KPIs and Binocs
All lab activities planned and scheduled in Binocs can be visualized via a range of standard and bespoke KPIs, data visualizations, and dynamic Gantt charts, as presented in Binocs Workspaces.
For instance, the below is an example of the standard Gantt Chart widget that allows work in a specific Demand Channel (workstream) to be presented in a dynamic report:
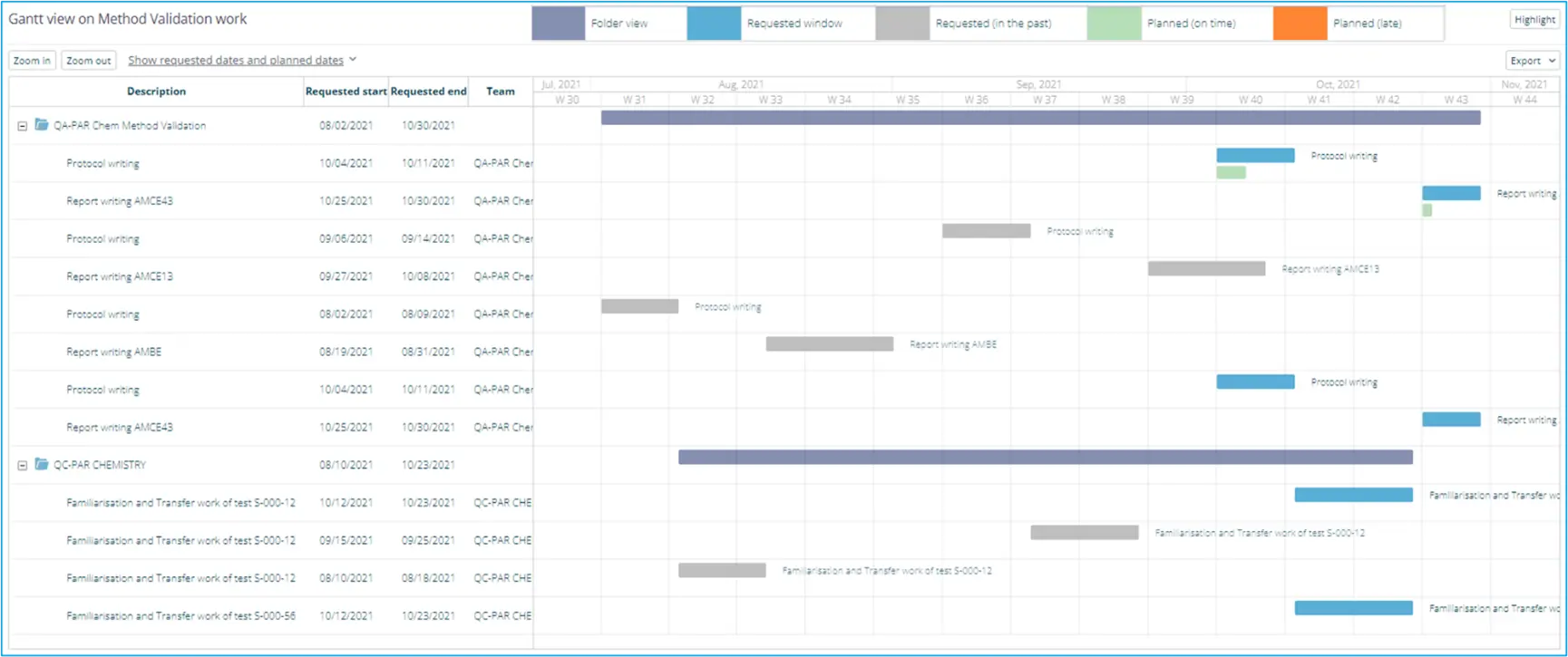
For Validation activities, KPIs and reports can be an excellent resource to allow your QA team to monitor and follow up on validation progress, providing an overview of past, ongoing, and future validation activities. Workspaces also represent the opportunity to define an interactive element in the lab’s VMP with visual metrics that can help to inform, refine and improve future master plans.
Demo you own your mission control center
Ready to launch your analytical method validation processes into orbit? Why not take a closer look at the Binocs system and request a detailed demo today?
Adam Lester-George
Adam has two decades of experience working in clinical trials, biomedical research, public health, and health economics, with a particular interest in the intersection between technology and life sciences. For 7 years before joining Bluecrux in 2019, Adam was the director of healthcare innovation consultancy “LeLan” and brings a wide range of insights to his role as Content Specialist for Binocs.



