The new meaning of lab digitalization in quality control labs
During the SmartLab Europe event (100% digitally this year), we were thrilled to meet with a lot of interesting people and exchange thoughts on building a roadmap for the lab of the future. After 15+ virtual conversations with a variety of attendees, our key takeaway is this: although the topic is not new, the sentiment around the lab of the future is evolving and maturing, and expectations around what the real benefits of QC lab digitalization actually are.
Lab of the future, an ever-evolving concept
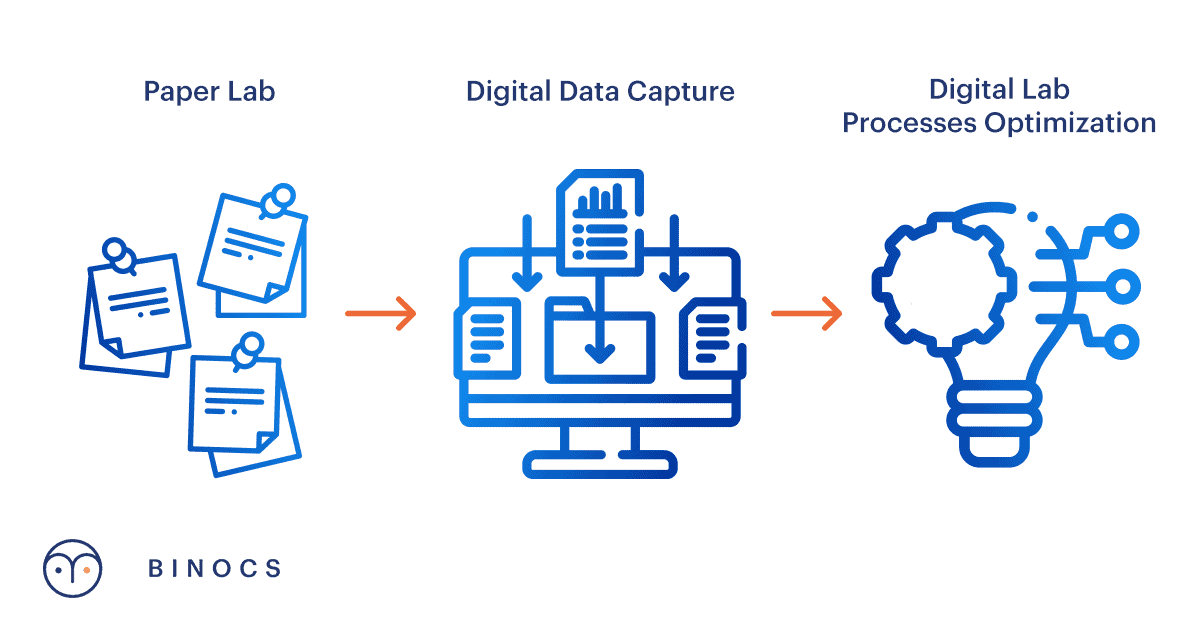
For people new to the idea, ‘digitalizing the lab’ sounds like a synonym for moving toward a ‘paperless lab’. But, with more and more companies having a lab digitalization strategy top of mind, we see the discussion moving beyond the visible outcome (=’paperless lab’) and more towards the overall value that digitalization could bring.
In one of our meetings, somebody summarized it beautifully: “the initial goal was to go paperless, but it’s about more than just that. The real value of digitalization is not that it replaces paper. It’s that it should make our processes more fluent, resulting in gains in efficiency and ultimately allow us to develop new medicine faster”.
In many of the other meetings, we heard different versions of the same story: seeing lab digitalization, not as a means to go paperless but rather to optimize process fluency.
Why we need to move beyond paperless
In a continuing effort to reduce costs and increase lab efficiency, many companies are looking to digitalize and automate laboratory processes, e.g. by going into more detail in LIMS and/or ELN. Such extensions can include capturing all method details (with or without a CDMS) and go as far as bidirectional instrument integration. It’s clear such digitalization helps to increase data integrity.
This makes the core business, what you deliver to your internal client, paperless. However, will it make everything in the lab itself paperless? Is the calibration plan integrated into those systems? Is method development included? And ultimately, will it help to optimize your lab performance?

Want to optimize your lab resource utilization?
Take your lab to the next level of operational excellence with our white paper:
5 principles to improve lab performance using Lean Lab strategies
The challenges of lab process optimization
Therefore, let’s think beyond just digitally capturing the information and switch our focus to process optimization. For example:
Hidden campaign rules
There are some hidden business rules in the testing work, e.g. how do we do campaigning? These rules are often undocumented, not digitalized, and let alone “automated”. Automating the campaigning process is an opportunity to not only minimize non-value-added work but to fully optimize throughput.
Unclear prioritization of work
Is the prioritization of work clear? What can we deliver by when? To answer this, we need two additional steps:
- What is the total picture of what lies ahead of us? Not all that info might be in the IT systems mentioned above. For example, do they contain and quantify calibrations work? Method development work? R&D support?
- Who can do this work and when?
The silver lining
And that brings us to the silver lining: yes, information might be ‘paperless’, but as long as everything remains unconnected, it brings little added value. Labs should therefore shift their focus and aim toward digital process optimization of the whole lab.
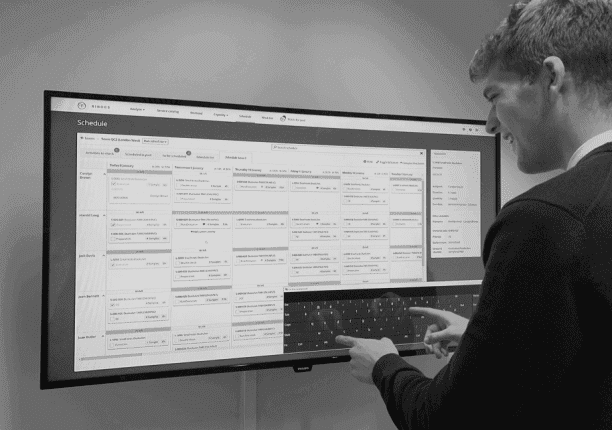
Planning & scheduling the digitally enabled lab
Let’s take an oversimplified example. Let’s say a lab supervisor wants to create a daily schedule for the QC team and needs four pieces of information to create the puzzle:
- What test methods do we need to do? Do we have the complete demand picture?
- According to which rules are we campaigning samples?
- Which lab analyst is competent to do which test method? This is typically known in LIMS, but LIMS isn’t able to plan the demand accordingly.
- What is the availability of our lab analysts? This is often known somewhere (HR system) but you can’t schedule as long as you don’t have the complete demand picture.
In the paperless lab (parts of) this information is stored digitally, but as a whole, it remains unconnected. This means you still need to rely on your lab supervisor’s undocumented expertise to connect the dots (manually campaign received samples, manually assign the work to the right analysts,… So, what’s the added value here?
Digital process optimization means you have a resource management system that connects information silos and automatically:
- Synchronizes received samples from LIMS.
- Assembles those samples into campaigns according to rules you can configure.
- Proposes a schedule based on competencies, due dates, constraints, and analyst & instrument availabilities.
Digitalization also makes QC labs resilient in turbulent times. Click here to learn how.



