Digital Transformation: a case for interoperability across Quality (part 3)
In our exploration of digital transformation (DX) and interoperability in Quality systems can streamline pharmaceutical release operations, we’ve highlighted the multifaceted challenges teams face and the broad spectrum of digital solutions available. Central to addressing these challenges is the implementation of an effective Resource Planning and Scheduling (RPS) system. As such, in this third and final part of this blog series, Adam Lester-George delves into how Binocs, as a specialized RPS, offers a vital digital resource for Quality teams, focusing on its specific applications and benefits within a comprehensive digital ecosystem.
Part 1: Quality must be greater than the sum of its parts
Read Part 1 of this series here.
Part 2: How can a clear digitalization strategy help to solve Quality challenges?
Read Part 2 of this series here.
Part 3: The strategic role of Binocs in Quality digital transformation
In the previous part of this series, I described the vital role of certain digital tools in revolutionizing modern Quality operations. In particular, I highlighted the use of LIMS (Laboratory Information Management Systems) and QMS (Quality Management Systems) which, while perhaps not ubiquitous, are certainly exceedingly common among contemporary pharmaceutical Quality Assurance (QA) and Quality Control (QC) operations. The reason for their popularity is clear: by using digital systems to manage complex or repetitive tasks, teams can significantly reduce the time investment, duplication of work, and risk of human error that typify purely manual processes.
LIMS and QMS are just two examples and there is, in fact, a wide range of systems that have potentially important roles to play in streamlining Quality operations. The true value of implementing such systems extends beyond their respective specialisms, to how they all interact as part of a broader digital ecosystem. As such, carefully mapping out such an interconnected network should be a consideration for any Quality team developing a DX strategy. Below is a simplified example of what such a system might look like, adapted from our white paper “Integrated QA for Pharma Release”:
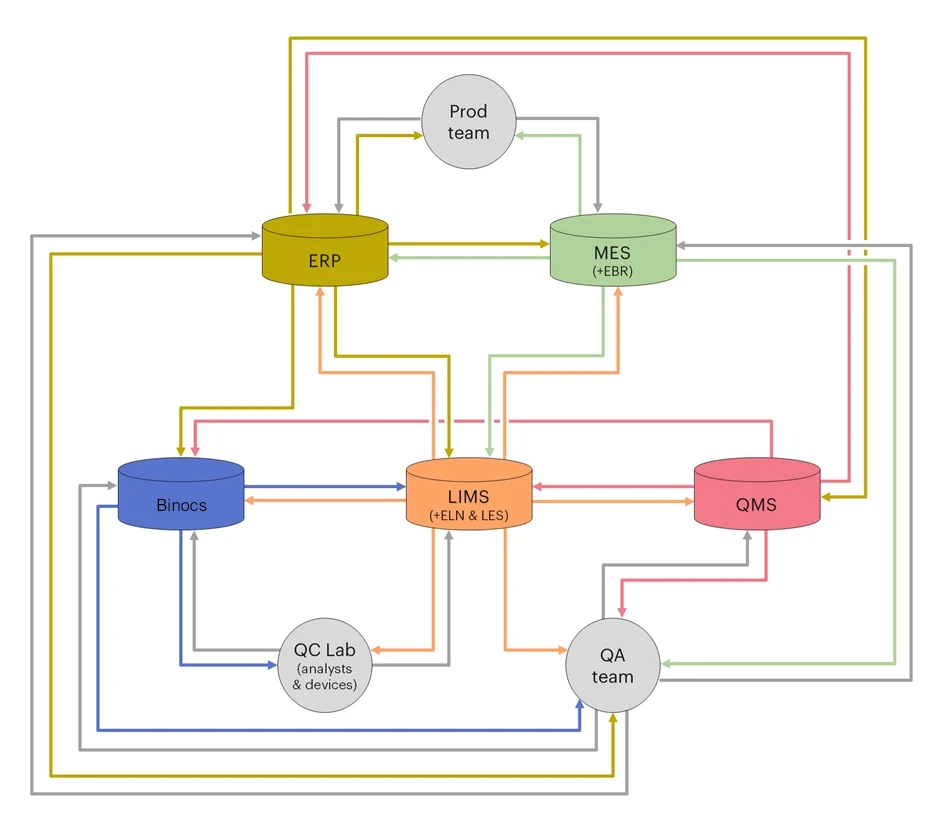
What I would especially like to highlight in this diagram is the fact that the five core systems (LIMS, QMS, Binocs, Enterprise Resource Planning system, Manufacturing Execution System) are both deeply interconnected and of comparable importance. Rather than considering these platforms as being in competition with one another, it is crucial for any successful DX strategy to present them as complimentary and as all contributing to an integrated system that is greater tham the sum of its parts.
That said, you may (due to budgetary constraints) be forced to choose just 3 or 4 of the systems for immediate implementation in your Quality department. Given the mainstream awareness that already exists concerning the other four systems illustrated above, allow me to identify a few of the specific benefits you can expect by choosing Binocs as part of your Quality DX plan:
- Intelligent Forecasting and Capacity Management: Binocs helps Quality teams manage the variability in QA and QC workloads by aligning available resources with demand, thereby mitigating capacity issues and addressing workload volatility.
- Resource Allocation and Scheduling: Binocs facilitates efficient allocation and scheduling of human and instrumental resources within both QA and QC functions, directly addressing the challenge of managing incoming workload and resource capacity. It ensures that tasks are assigned based on skills, qualifications, and availability, optimizing resource use.
- Integration with Key Quality Systems: Binocs plays a crucial role in the interoperable digital ecosystem via its system agnostic, zero-code integration that allows it to connect seamlessly with other critical platforms such as LIMS and QMS. This therefore facilitates smooth information flow and efficient scheduling of Quality tasks.
- Enhancing Visibility and Oversight: providing QA with complete oversight of end-to-end QC operations is vital, and Binocs enables this by offering visibility into the progress of ongoing work. This allwos QA teams to anticipate batch release readiness and manage QC oversight more effectively, helping to mitigate bottlenecks and unexpected volatility.
The integration of Binocs into the Quality operations digital ecosystem represents more than just the addition of a tool; it signifies a strategic move towards a more interconnected, efficient, and proactive Quality function. This RPS system’s value is in its specificity—targeted at non-GxP aspects of Quality operations, it complements GxP-compliant systems like LIMS and QMS, creating a holistic digital landscape that transcends traditional QA/QC boundaries and connects with the wider supply chain. And, being a non-GxP system, it is significantly faster and hassle-free to implement!
As outlined in our white paper, the implementation of Binocs within a well-planned digital transformation strategy is essential for pharmaceutical companies aiming to enhance their Quality operations. It illustrates how an integrated approach, leveraging the unique capabilities of systems like Binocs, is not just beneficial but essential for a future-ready, agile, and high-performing Quality function.
We invite you to download the white paper for an in-depth exploration of the topics covered in this series, and to discover how your organization can achieve a seamless and robust digital ecosystem that elevates both QA and QC functions to new levels of excellence.
You can download your free copy of the paper via the below form now!