Analytical Method Validation: it’s not brain surgery (but it might be rocket science)
Since both Richard Branson and Jeff Bezos have recently entered orbit, Adam Lester-George explores what commercial space flights can tell us about QC analytical method validation.
Major Gagarin to Ground Control
Across the centuries, dreamers everywhere have sought to reach for the heavens. In practice, there are many obstacles to humanity touching the stars, the biggest and most significant hurdle being the act of escaping the Earth’s gravity – to paraphrase Newton, “what goes up must come down… unless you have rockets!”.
In April 1961, Soviet cosmonaut Yuri Gagarin was the first human to make this dream a reality when the Vostok 1 rocket launched him into orbit around the Earth.
It requires a huge amount of energy to lift a payload into space and lots of rocket power means lots of rocket fuel, lots of highly calibrated control systems and nerves of steel! For all the bravery and skill that Gagarin displayed on that fateful day, there was an unseen army of pioneers working away behind the scenes to ensure that everything ran smoothly.
Being strapped on top of a 5-ton rocket, filled with volatile fuel and accelerated to 40,000km/hr, Gagarin must have been thankful for the comprehensive QC that was in place around every aspect of this remarkable event! Indeed, not only was each component rigorously tested before assembly and every inch of the rocket inspected before launch but there were also thousands of processes that needed to be checked and validated. Everything from the ignition sequence to the atmospheric systems and Gagarin’s own communications responsibilities needed to be defined, reviewed and practiced months in advance, based on limited tangible experience and carefully considered scenarios.
Refining the future
It’s been 60 years since this momentous occasion yet, were you to compare images of that seminal launch event with a 2021 launch of the SpaceX Falcon Heavy rocket, it might at first appear that very little has changed.

In fact, the intervening decades have witnessed the iterative refinement of methods and, despite superficial similarities, things couldn’t be more different beneath the surface. For one, today’s space-faring missions have a lot more historical data upon which to base their processes for crewed space flights. This has led to much greater standardization in approaches, which has, in turn, paved the way for the commercialization of space travel.
For instance, ahead of the first human spaceflight in history, the Soviet space program had only launched 24 rockets, of which a full 12 had ended in failure. This 50% success rate allegedly gave Vostok 1’s Chief Designer chest pains during the rocket’s launch countdown. By comparison, today, SpaceX’s launch success rate for all rockets (even those not designed for crewed flight) exceeds 95%!
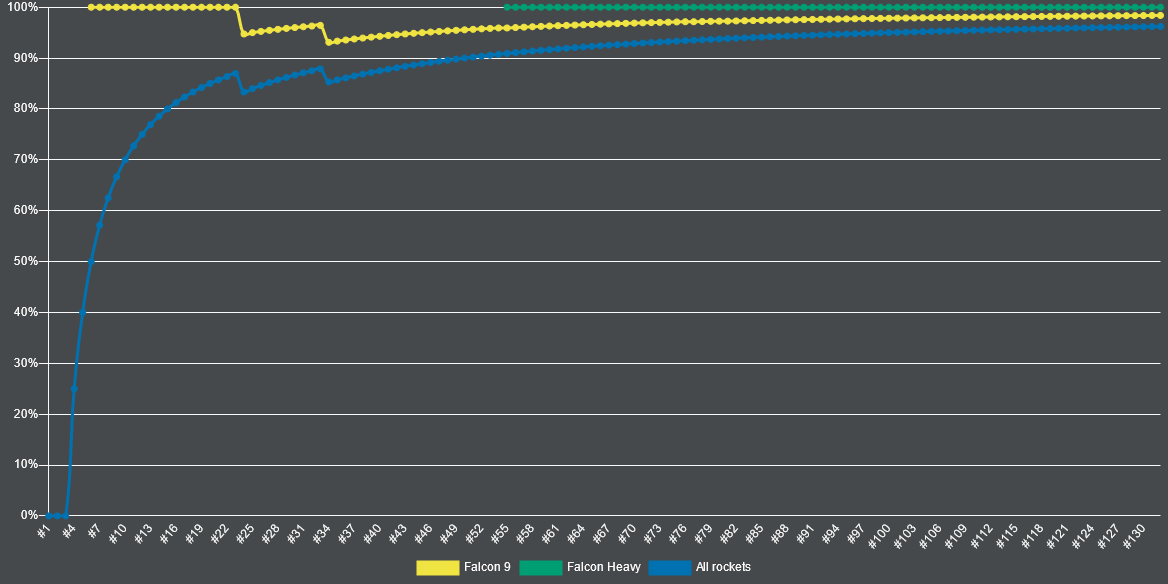
The Principle of Repeatability for analytical method validation
“So what”, you are probably asking yourself, “does space exploration have to do with analytical method validation?!”
Well, just as with rocket launches, the results of pharmaceutical analytical procedures must be reliable, accurate and reproducible – you want to guarantee you can trust the product and thus you must have absolute confidence in the quality of the analysis process.
One key to the success of modern rocketry is the principle of repeatability – when each commercial launch costs upwards of $150m and potentially hundreds of lives are at risk, it is critical that all systems and processes can be trusted. This means testing for and documenting all of the characteristics of a launch scenario to ensure that a mission can be successfully delivered in a predictable way every single time.
Launch technicians’ responsibilities extend far beyond managing systems during and after take-off; indeed, much of the rocket team’s time is spent defining and testing processes, modeling the launch conditions, documenting appropriate methods that have been refined as a result of decades of practical and theoretical work, and ensuring that every eventuality has been accounted-for.
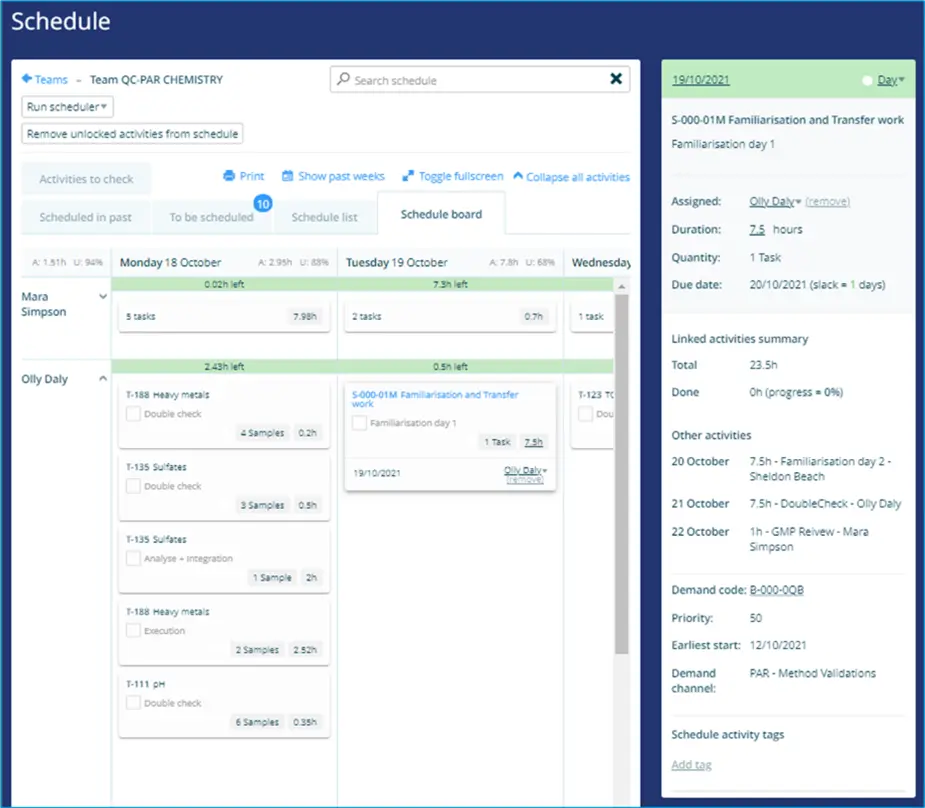
This same cycle of designing, qualifying and refining processes is exactly what has helped to streamline and accelerate QC lab functions in recent years. Quality control for pharmaceuticals is critical, not simply because of the investment in production but, crucially, because far more than a few hundred lives are at risk if something goes wrong.
Expertise is key
In rocketry, all of the preparatory work is designed to limit variability and increase repeatability across launches by defining, in advance, those characteristics that are critical to success. For instance, through repeated modeling and continuous validation, launch engineers will be able to predict ahead of time:
- The accuracy and error margins inherent in the launch model compared with reality
- The precision of launch models across bootstrapped testing
- The acceptable range of environmental conditions for a safe launch
- The robustness of launch parameters across the range of acceptable conditions
- The specificity of processes that must be followed for a given rocket type
- The correct linearity in the fuel-to-payload correlation to deliver an optimal launch
- The safe limits for detecting and quantifying potential problems
The responsibility for defining and testing all of these factors does not rest with one specialist group but, rather, is distributed across the expertise profile of the wider team and the relevant work must be carefully but dynamically allocated to the appropriate staff and then clearly summarised in SOPs and detailed launch protocols.
In QC labs, a similar set of validation characteristics must also be clearly defined to ensure the repeatability of analytical methods:
- Sample tests must demonstrate an agreed degree of accuracy compared with true values
- They must adhere to an acceptable level of precision, with a reliable consistency in test results across identical samples and standards
- A clear range must be defined for upper and lower bounds of detectability in samples
- Analytical procedures must demonstrate robustness, with result reliability under different conditions
- There must be selection certainty, wherein tests are able to measure analytes with a high degree of specificity, even in the presence of other substances
- Tests must demonstrate linearity, such that results are always proportionate to the concentration of the sample
- The detection limit, which defines the smallest quantity of a substance that can be detected by a test (present vs absent)
- The quantification limit, to specify the smallest quantity of a substance that a test can reliably measure
As with launch processes, analytical method validation must also be fully documented by a range of contributing specialists. For instance, the Validation team may write the Protocol (process design) but Lab specialists will perform the familiarization test runs and validation runs (process qualification), before passing back to the Validation team for the report write-up (continued process validation).
This cycle of work must be planned for the relevant team members at the appropriate time.
Planning for success
Despite working to strict launch schedules, commercial space flight planning is nonetheless somewhat “lumpy”, with tasks being clustered without a flat workload that can easily be netted from gross capacity.
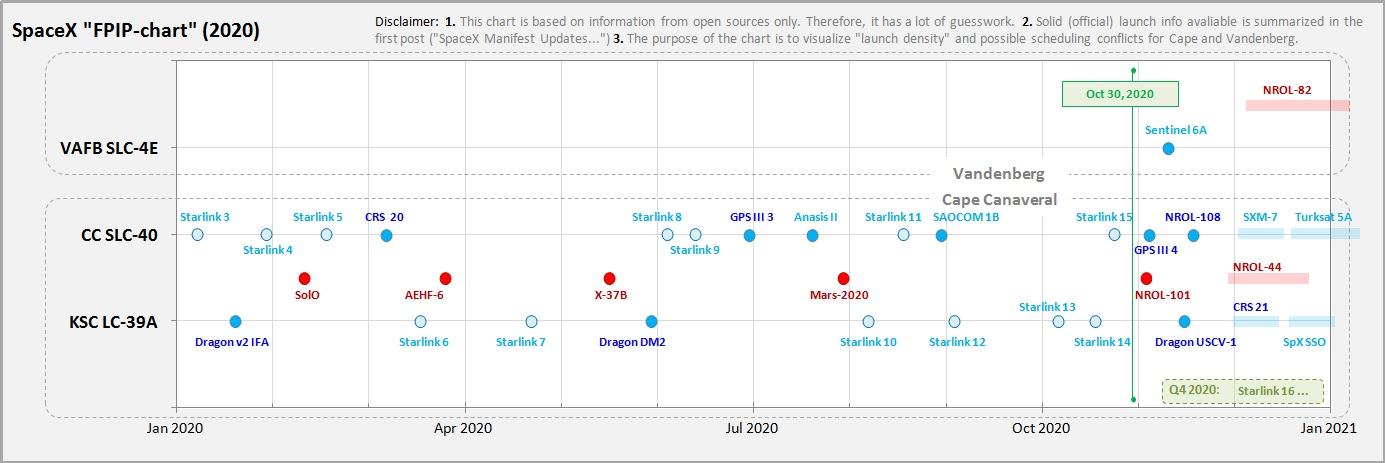
As shown in the above chart of commercial SpaceX flights, all of the highly specialized work from multiple expert teams must be planned around client (NASA) requirements, meaning that all of the critical validation process management must be carefully and dynamically scheduled so as to not interfere with safety protocols or to compromise business commitments.
In general, QC planning is similarly lumpy, with method validation demands arriving in clusters that must also fit around pre-defined stability testing schedules and the need to undertake routine lab maintenance, equipment calibration, and other tasks requiring a variety of qualifications and skills.
As with the specialists working to deliver successful space programs, QC labs are also responsible for more than just the ongoing analysis of samples (e.g. “launch day” activities). In fact, as much as 40% of lab time is dedicated to this other work, foremost among which is validating the analysis methods themselves.
Such validation work can be triggered in a variety of different ways, including:
- Periodic re-validation
- New product introductions
- Changes to test methods and equipment
- Tech transfer
- Test bridging between labs
All of which must be scheduled around other commitments.
Sending your QC lab resource planning into orbit
Organizations such as SpaceX, Blue Horizon and Virgin Galactic all work to demanding timelines on highly public-facing and exotic projects with huge numbers of moving parts (literally and figuratively) with the goal of delivering results to clients on time, at cost and with an extreme level of quality. While each company may plan their work differently ahead of launch, on the day that a rocket is sent into space, each nevertheless requires a mission control center to effectively assemble all of the diverse factors for effective results.
But why should mission control centers be limited to NASA? Balancing all of the critical elements of a modern QC lab can be even more impactful than sending humans to Mars and seamlessly integrating your team’s method validation responsibilities with their value-add work needn’t feel like shooting for the Moon.
Thankfully, Binocs resource planning and scheduling have you covered!
Why not take a look at our demo page to learn more and keep your eye out for our follow-up blog where we’ll discuss the specifics of how to use Binocs to manage your analytical method validation.

Adam Lester-George
Adam has two decades of experience working in clinical trials, biomedical research, public health, and health economics, with a particular interest in the intersection between technology and life sciences. For 7 years before joining Bluecrux in 2019, Adam was the director of healthcare innovation consultancy “LeLan” and brings a wide range of insights to his role as Content Specialist for Binocs.