
Handshake integration: bringing Quality into end-to-end decision making
At Bluecrux, we believe that Quality Control teams represent a critical but undervalued stakeholder in the end-to-end pharmaceutical product release planning process. In this article, I outline our vision for “handshake integration”, our digitally enabled approach to improving communication between QC and other stakeholders and facilitating improved lead times across the entire E2E flow.
In a rush? Here are the 3 key takeaways
- 👉 Quality teams represent a critical stage in the product release process but are rarely involved in S&OP discussions, despite accounting for up to 55% of end-to-end lead times
- 👉 Evidence demonstrates that lead time savings can be achieved by improving the interaction between Quality operations and other key stakeholders, such as Manufacturing and Supply Chain
- 👉 Bluecrux has developed a digital solution that allows APS, QC scheduling, and digital supply chain twin technologies to interface and facilitate dynamic, priority-based lead times to be automatically recalculated for exceptions
Time is money
In any commercial operation, time is money. Pharmaceutical manufacturers are acutely aware of this fact and therefore constantly strive for new ways to shave days from their end-to-end manufacturing processes. For biologics in particular, the time from initial manufacturing run to final release can easily take over a year; every lost day during this process is a day that orders are not being fulfilled and the product is unavailable to patients.
Quality operations (QC testing and QA release) are critical to pharmaceutical order fulfillment, however Quality lead times can be significant. Indeed, data from Axon (our digital supply chain twin SaaS solution) suggests that QC processes can account for up 55% of the total end-to-end lead time, with up to 45% of inventory being tied up in testing at any given moment! As the last stage in the product release process, delays in QC/QA have the potential to substantially impact overall manufacturing throughput, with significant consequences for carrying costs and working capital.
The fact that Quality Operations represent such a significant stage in the manufacturing release process also means that they are a potential source of efficiency gains. Many of the internal customers from other stages of the E2E flow may, however, simply not view Quality (and in particular QC) as active participants in product release planning. Indeed, Supply Chain professionals referring to their APS will often see the time from product completion to release as a single, often inaccurate figure (the Goods Receipt Processing Time), without significant consideration of the various, complex Quality steps this comprises. This complexity is what contributes to long lead times and results in a general sense that the QC department is something of a black hole that eats time in ways that aren’t fully understood and which cannot be resolved.
With modern technology, however, we contend that Quality teams can be more flexible and dynamically accommodate changing demands, and that communication between them and the rest of the E2E stakeholders can be significantly improved. Below, we outline our vision for how digitalization can help QC to be better integrated into supply chain planning decisions and can therefore significantly contribute to achieving additional efficiency gains.
Misconceptions about Quality operations
Despite its vital role, representatives from the Quality department are rarely invited to the table when discussing supply chain planning and execution optimization. There are three frequently cited reasons for this omission:
- Some argue that Quality is a separate organizational branch and should be segregated from decision making to protect regulatory compliance;
- Others point to the personality differences between Supply Chain ‘engineers’ and Quality ‘pharmacists’, claiming that being “wired differently” would create unnecessary tension;
- It is also often argued that Quality is simply a support service and its contributions are not required since any problem encountered in the supply chain can be accommodated in QC by adding more analysts and equipment.
The last perspective, in particular, reflects a commonly held misconception about the nature of Quality operations. Upstream stakeholders in the end-to-end drug production flow often state that QC and QA processes must be dedicated to the Production facility and therefore scheduled and executed linearly as part of that whole flow. From this point of view, QC and QA are considered sequential steps that are no different from operations on reactors, filling lines, and packaging lines.
The true complexity of QC
Unlike production equipment, however, a QC lab handles a substantially greater level of operational complexity. Most current QC labs are organized around clusters of test methods and testing technologies, many of which can be highly complex and require skilled and qualified operators. The below diagram illustrates a typical QC lab organization and its connection with Production and Supply Chain operations:
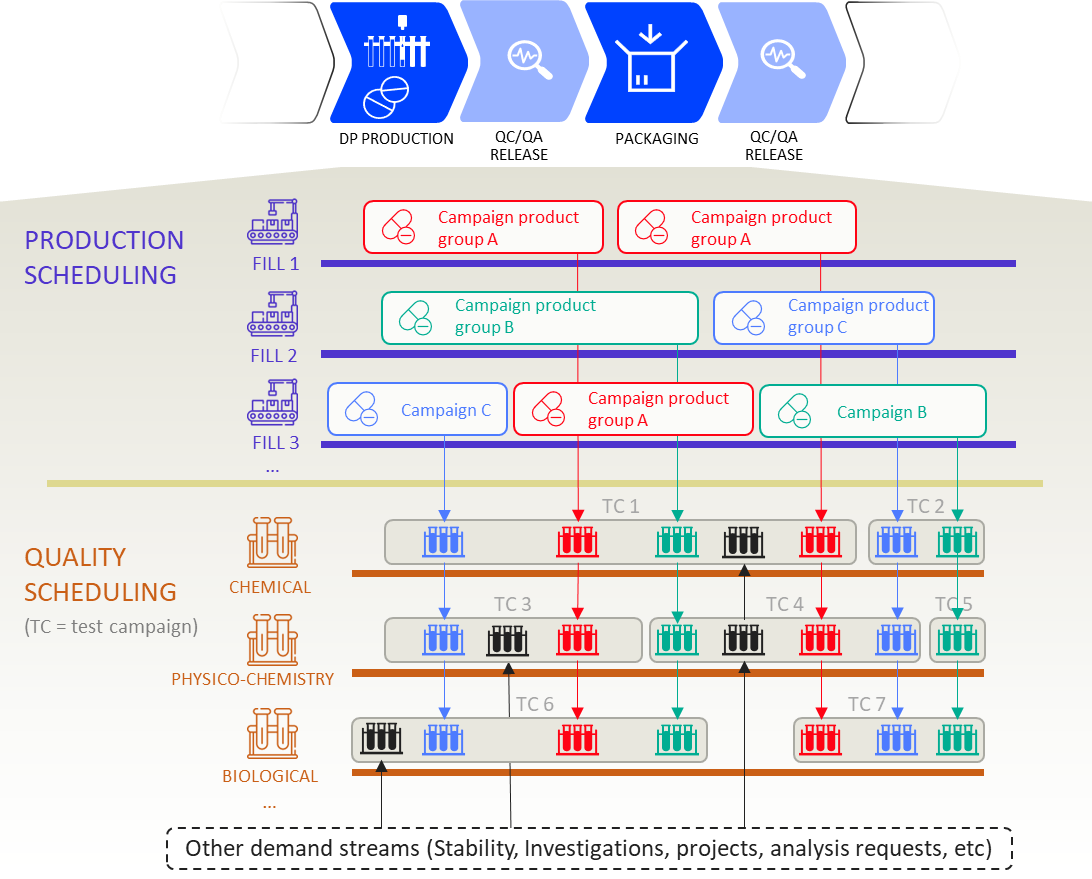
In this format, a QC lab performs release and stability testing for a range of products manufactured at nearby sites and production facilities. Samples are taken at every stage of the production process and then submitted to specific labs—such as the Analytical Lab or the Microbiological lab; other samples may be tested on the production line by an IPC testing lab. Additional testing may be outsourced to contract organizations in periods of low in-house capacity or if specific expertise is required.
There are two main drivers of complexity in QC operation:
- There are many different samples requiring a range of different tests, each reliant on test methods that must be executed by skilled operators (who must be carefully coordinated);
- A large number of test methods use instruments (such as HPLCs) where multiple samples can be tested simultaneously in runs, which can represent a significant efficiency gain but it is far from being a linear process.
Combined, these points represents a transverse operating model for testing a production batch, a reality that is at odds with the simplified expectations that might be held by stakeholders in other departments—and this example only considers the situation for testing one production batch!

In reality, hundreds or even thousands of test requests from different sources must fit into the lab schedule, with campaigns composed of samples from multiple production batches and other demand sources entirely (such as stability studies and specific test requests). This all results in Lab Planners needing to become masters at playing a giant, ever-changing game of Tetris. As the operational complexity of Quality teams increases, this game gets exponentially harder to play without additional support—and service level adherence is the first thing to suffer.
Reducing Quality lead times: an early case study
It is clear, therefore, that Quality operations are complex and there are various reasons why the lead times can be sizable. Despite this, we contend that there must be a way of streamlining the process to shave precious days off the turnaround.
In our early days of consulting on pharmaceutical supply chains, we were commissioned to conduct a lead time reduction project at a mid-size vaccines manufacturer. They had a central QC and QA department supporting four bulk and formulation facilities, and a packaging line. Long and variable lead times for individual operations are an inherent characteristic of biologicals manufacturing but, even in that context, the cumulative effect across the Quality function was excessive. Upon investigation, everything pointed to one principal root cause: the interaction between Quality operations and Production.
In the first instance, we advised the immediate institution of a weekly review meeting for each production facility between Production, Procurement, Distribution Logistics, and QC/QA, supported by Excel follow-up tools and dashboards. The below chart shows the lead time breakdown for the QC testing and QA review operations for a chronologically ordered list of production batches, with a clear “before and after” effect visible. What we had implemented was effectively an S&OE (Sales and Operations Execution) process years before the concept had been popularized.
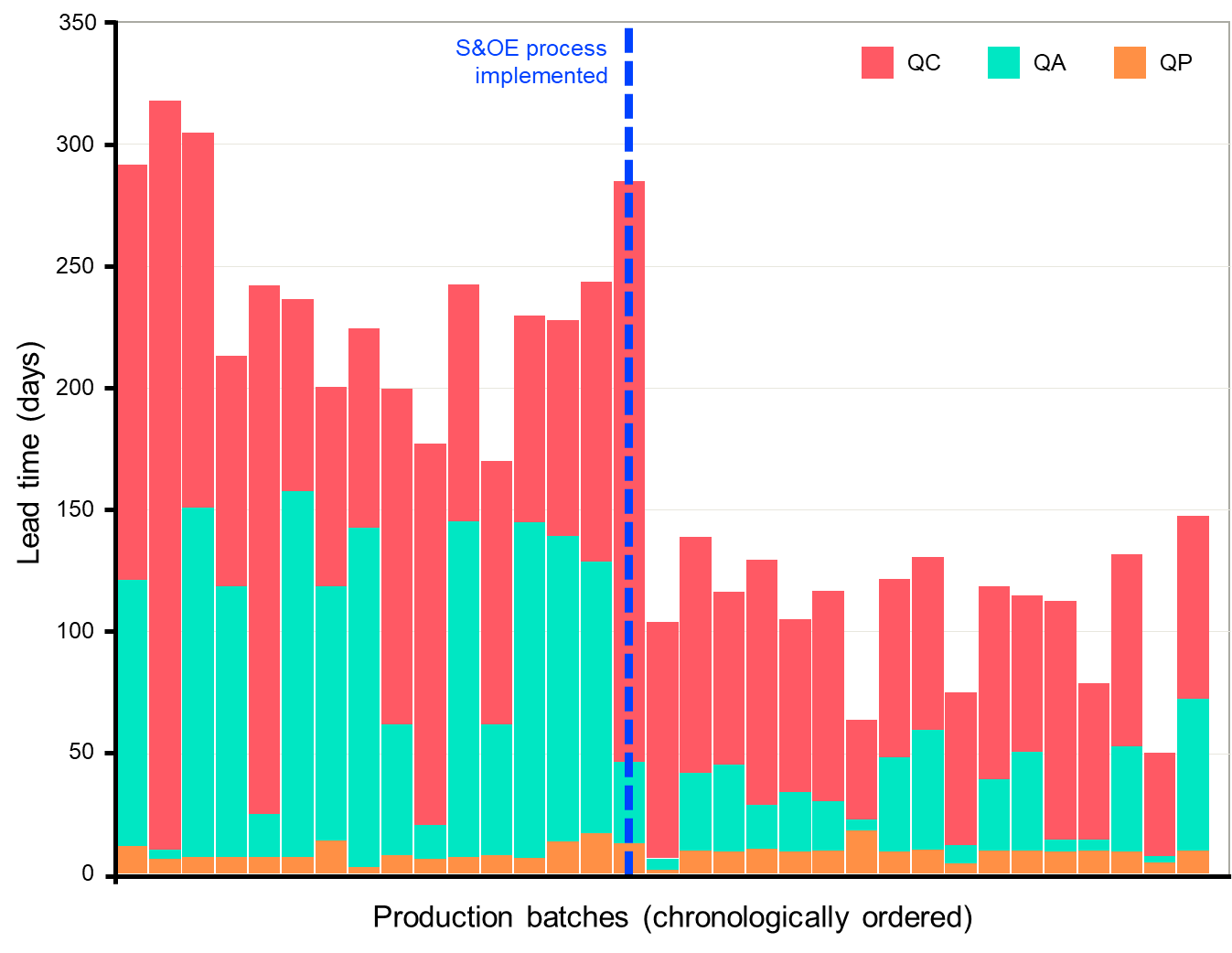
Although the results were impressive, there were some major concerns:
- For one, the process was entirely paper and Excel-based and the data upon which the decisions and actions were based was incomplete and not fully reliable;
- Further to this, collecting and preparing the data represented a significant effort, with a weekly a race against the clock to maintain the cadence, therefore threatening the sustainability of the process (and any tangible savings that might have resulted);
- Additionally, there was no effective S&OP and Production scheduling system in place (which could have otherwise avoided many of the supply chain problems) an a very limited production planning tool (which did not account for Quality operations).
While a step in the right direction, the S&OE process we established in this example was not equipped to handle the full range of problems, especially considering the full complexity of QC operations as outlined above. It is therefore clear that such processes must be supported by more than whiteboards and Excel tools. The solution is digitalization.
Digitalizing Quality operations
It is evident that reducing the complexity of QC operations simply isn’t an option. As described, Quality labs are high throughput environments that sit at the nexus of demands from a range of different stakeholders and act as gatekeepers for drug release. This complexity is a feature, not a bug, an emergent characteristic resulting from the pressures placed upon the Quality function. As such, rather than seeking to simplify operations, the complexity must be embraced and managed and this can be achieved using advanced digital solutions—data-driven decisions, powerful algorithms, AI, and robust operating models and standards governance.
One example is digital scheduling: QC labs can readily employ advanced algorithms and business rules to manage some of their planning complexity but the approach will be substantially different from Supply Chain scheduling systems. For instance, QC scheduling is focused on personnel and instruments, with no consideration for material flow. This means that planning algorithms must account for analyst qualifications and accommodate employee well-being (e.g., by rotating tasks to reduce routinization).
Another example is digital business rules: while the primary goal of an effectively operating QC team is product release, labs receive demand from various other sources such as stability studies, validation, and qualification support (methods, cleaning, instruments, reagents), method transfer, R&D support, import release testing, etc. In some labs, the auxiliary test demand might come in the range of 40%. This added layer of complexity makes it inevitable that there will be competition between batches to obtain test results within a requested service level. This is why QC labs seek to manage expectations and agree on service levels with their internal customers a priori (e.g., the lab will do their best to deliver within 20 days), with internal decision and escalation plans for when delivery deadlines cannot be met. Consequently, these plans must be built into the operating model and that’s where algorithmically enhanced test prioritization and re-allocation rules can be used to reduce complexity and facilitate greater transparency.
Thus it is clear that Quality teams have their own priorities and technological requirements that are distinct from—but complimentary to—the processes used by other teams in the end-to-end flow. How, then, should these distinct but interconnected approaches be combined to reduce lead times and facilitate overall service delivery?
Handshake integration: the connection between Supply Chain and Quality
Regular integrations simply allow data to be directly exchanged between two applications, job done. With “handshake integrations”, however, data is exchanged in multiple cycles and layers in a process akin to negotiating the terms of a contract: have both parties agreed on the objectives? Has the agreement been translated into the appropriate legal language with terms that are in-line with local laws? Is there agreement on the financial consequences for all the possible scenarios? Only when all the lights are green does the handshake (and signature) take place.
What does this look like for a QC lab? Let’s take a simple use case: a batch should be released five days earlier than initially planned or agreed because of inventory issues. Here’s a typical analog flow for this scenario:
- Supply Chain detects an inventory issue;
- Supply Chain investigates and concludes that advancing the release of a batch is the best option;
- Supply Chain asks QC for this exception;
- QC evaluates the question (and let’s suppose they agree);
- Supply Chain and QC shake hands;
- QC and Supply Chain adapt the batch due date to ensure proper execution of the decision.
Often, step 3 in this sequence is addressed less as a request than a command, with Supply Chain stakeholders expecting QC to execute testing without clear visibility or understanding of their capacity to deliver to changing priorities.
The digitalized version of this process involves integration between an APS, a digital supply chain twin, and a QC scheduling solution and instead looks like this:
- The APS automatically detects an inventory issue;
- The APS queries the digital supply chain twin to identify a differentiated lead time based on the batch priority. If the batch must be expedited, a new due date is recalculated based on the shortened lead time, and it is then marked as an “exception”;
- The QC scheduling system receives the updated and prioritized request;
- The QC scheduling system’s algorithm shows that the new due date is possible;
- QC sends the updated data and confirmation to the APS;
- All three applications support the proper execution of the decision.
If the APS algorithm cannot solve the supply chain glitch or the QC scheduling algorithm cannot accommodate the shortened lead time, then additional human intervention will still be needed to make decisions that the digital systems cannot yet handle (such as stock balancing between sites or organizing overtime in QC). This is where digital integrations meet the business process world.
By digitalizing the process in this way, 90% of the effort is now shouldered by automated systems, with minimal manual intervention required. In addition, the algorithmic solution supports informed decision making and facilitates negotiations between QC and Supply Chain because the likelihood of delivery can be calculated, and the QC team can decide whether to accept the exception based on its estimated impact on other batches.
The above scenario is, of course, just one single case. In practice, there will be dozens or hundreds of cases that need attention, each with distinct considerations. For instance, different cases will have different priorities (how soon is the decision required), locations (which production line or lab), seniority requirements (who needs to make the decision), and other characteristics (is it one-time or recurring, what is the severity of the issue and the scale of the impact).
Given all these moving parts, the process is a shoo-in for automation—and we have the solution! If you’re interested in learning more about Bluecrux’s vision for integrating QC into decision making around wider E2E planning, reach out today.
Get in touch
Check out some related content
-
What are QC networks and how can they help to optimize global quality operations in life science organizations? Everything you need to know!Read more
-
Collaboratively Planning Quality-Control Laboratories with Binocs
QC Laboratories Planning, which is what? Today, QC labs have to be lean labs. While coping with complexity and volatile demand, QC labs are constantly asked to increase analyst…Read more -
Using a data lake to integrate supply chain with QC
GlaxoSmithKline, one of the top 10 big pharmas globally, uses Binocs to slash release times, enhance utilization & connect Supply Chain to QCRead more





